WHAT IS IT?
Spill some, and you'll probably throw a pinch of it over your shoulder to ward off bad luck. In parts of Africa, 23 Kg (50 pounds) of it is enough to get you a wife. Lack of it lost Napoleon an empire. Escoffier himself couldn't cook without it. A major meat packer uses 31.5 t (35 tons) of it a day. For centuries, it has been one of the most valuable substances known to man. You probably know it as common table salt. But just what is that white stuff in your salt shaker?
Salt is a mineral composed of sodium and chlorine. Neither can exist alone in natural form. Together, they form the stable chemical compound that is sodium chloride, or salt. To geologists, salt is known as halite, from "hals", the Greek word for salt. Halite belongs to the family of minerals which are compounds of the metals with the halogen elements fluorine, chlorine, bromine, and iodine.
Salt is a crystal of the isometric system, which means it is usually cubic and perfectly symmetrical. Pure salt crystals are colourless, although a pile of them will appear white. Impure crystals may be various shades of yellow, red, blue or purple. Salt is one of the earth's most abundant minerals. The world's oceans alone contain an estimated 18,756,000 Km3 (4,500,000 cubic miles) of salt, or enough to cover the entire surface of the globe to a depth of 122 m (400 feet). Millions of tons of salt are extracted each year from the sea and underground deposits.
Salt is such a basic commodity and is used in so many different ways that a chart of salt consumption is almost a barometer of economic conditions. From such charts, economists can trace the rise and fall of national prosperity.
Today, we can find over 14,000 uses for salt in industry, in medicine and in the home. In addition to its direct use in industry, salt plays a vital role as a raw material in the manufacture of other chemicals. Farmers depend on it to keep their stock healthy. Salt adds flavour to almost everything we eat and saves lives in winter by improving the safety of our highways.
Our plentiful supply of salt means that its value must be measured in terms of its versatility. Yet, in times past, salt was so highly prized it was used, like gold, as a medium of exchange. Our early ancestors soon learned that man, like the animals, needed salt in order to live. Ever since, not only our food, but our commerce, our politics, our religion and our superstitions have been flavoured with salt.
Primitive tribes valued salt so highly that coins were made from it. The natives of Africa's Sierra Leone were willing to sell their wives and children for salt. An oppressive salt tax was the final spark that kindled the French Revolution. Many of the world's wars have been fought for strategic salt supplies. During the American Civil War, the men and horses of General Lee's cavalry were decimated by disease because of a salt shortage that affected the future of the entire Confederacy. Salt was the reason for the construction of one of the most famous military roads in history, the Via Salaria, or Salt Road, between the salt works at Ostia and the City of Rome. The Roman legionnaires who guarded this route received a part of their pay in salt. From this ration, the "salarium argentum", derives our modern word "salary". Even today, a good employee is said to be worth his salt".
In many ancient civilizations, salt had great symbolic value. Because of its acknowledged power to purify and preserve, salt spilled on the parchment was a guarantee of good faith in signing a contract or making an agreement. Among the Greeks and the Turks, to eat salt with a stranger was a token of friendship. The Greeks made thank-offerings of salt to the gods they worshipped as givers of life. The ancient Jews offered salt to Jehovah at harvest time. In Jewish tradition today, bread and salt are the first things to be brought into a new house.
In some areas of the far East, people give their children little bags of salt to hang around their necks as protection from the "evil eye". And in parts of Russia no bride and groom would enter a new home without first throwing salt in every corner to protect them from harm and to encourage health and happiness.
Even in modern Christianity, salt has symbolic significance. The Bible contains many separate references to salt. In the ritual of baptism, salt is used as a symbol of protection against evil, representing purity of mind and soul and the power to resist temptation.
According to ancient superstition, when you spill salt, you risk losing the power to triumph over temptation and must banish evil spirits by throwing a pinch of it over your left shoulder.
The association of spilled salt and the appearance of evil is illustrated by the overturned saltcellar of the Last Supper. Judas, who has succumbed to temptation, is identified by Christ through the evil significance of the spilled salt. Which brings us back to the salt on your table. How did it get there? Where does it come from?
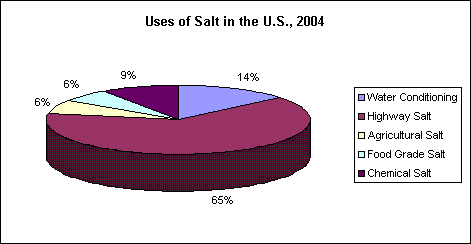
Salt is used in greater quantities and for more applications than any other mineral. It has more than 14,000 known uses. Everyone uses salt, directly and indirectly. Americans each consume more than 16 tons of salt during their lifetimes, 402 pounds a year for each living American. Only a small percentage of that massive amount is ingested as food. Last year, here's how usage breaks out among major uses:

The greatest single use for salt is as a feedstock for the production of chemicals. The chlor-alkali industry uses salt, primarily as salt in brine from captive brine wells, to produce chlorine and caustic soda. Demand for salt in to produce chemicals fell from 25 million metric tons in 1974 to a low of 16.7 million metric tons in 1992. However, chemical use rebounded in 1994 to 18.4 million metric tons. Much of the decreased demand for chlorine was attributed to environmental concerns about dioxins. Salt is also used to make sodium chlorate and metallic sodium by electrolysis and, sodium sulfate and hydrochloric acid by reacting with sulfuric acid.
All animals, humans included, require both sodium and chloride for life and health. Since the body cannot manufacture either, they are "essential" nutrients. While developed countries dedicate most of their salt to chemical production, developing countries often use most of their salt for human and animal nutrition.
Livestock, poultry and other animals do not always receive adequate amounts of sodium and chloride from forages and other feeds. They need supplemental salt as part of a nutritionally balanced diet to remain healthy, disease free, and to achieve optimum growth and reproduction rates. Because animals have a natural, definitive appetite for salt - they will eat only a certain amount - it is used to ensure adequate intake of less palatable nutrients and as a means of limiting feed intake. Salt can be mixed with feed or fed free-choice, and is an excellent carrier for trace minerals, It is produced plain or as trace mineralized salt, in 50 lb blocks, smaller spools, and as loose salt, commonly known as mixing salt.
Salt is the most effective, readily available, and economical highway deicer in use today. It assures winter driving safety and continued mobility in snowbelt states, even under the most adverse snow and icing conditions. Rock salt and solar salt are used on U.S. highways. In Europe, because of its availability, evaporated salt is sometimes used. The lowest temperature at which sodium chloride will melt ice (the eutectic point) is -21.12?C (-6.02?F), at a concentration of 23.3% NaCl. Salt works best at temperatures near 0?C (32?F) because melting occurs quickly with a relatively small amount of salt. As the temperature falls, it takes more salt and more time to melt ice because a higher concentration of brine is required. Fortunately, most snowstorms occur when the temperature is near freezing, where salt is very effective. More than 40% of the dry salt produced in the United States is used for highway deicing.
Water is considered hard when it contains calcium and magnesium (hardness ions). Hard water requires more soap and detergent for laundering, cleaning and bathing because suds do not form as well in hard water. The reaction between soap and hard water results in a greasy, curd-like deposit which makes fabrics feel harsh and leaves water spots on dishes and utensils. Mineral scale builds up in hot water appliances and industrial boilers, reducing energy efficiency and shortening appliance and equipment life. Water is conditioned or softened by removing the calcium and magnesium ions from hard water and replacing them with "soft" sodium ions. Water softeners use cation exchange resin to exchange sodium for calcium and magnesium. As supply water flows through the resin bed, the exchange takes place and the water becomes soft. Water softener cation exchange resins are regenerated with a 10% salt brine solution made by dissolving water softener salt.
Pulp & Paper. Salt is used to manufacture chlorine and caustic soda. In paper making, caustic soda is used to process wood fibers and chlorine is used to bleach the pulp. Sodium chlorate, also made from salt, is replacing chlorine as the primary chemical for bleaching pulp.
Other Industries. Salt is used to fix and standardize dye batches in the textile industry; it is used in metal processing and secondary aluminum making, to remove impurities; rubber manufacturers use salt to separate rubber from latex; salt is used as a filler and grinding agent in pigment and dry-detergent processes; ceramics manufacturers use salt for vitrifying the surface of heated clays; soap makers separate soap from water and glycerol with salt; oil and gas drillers use salt in well drilling muds to inhibit fermentation, increase density and to stabilize drilling in rock salt formations; hide processors and leather tanners use salt to cure, preserve and tan hides; and there are more.
Cloud Seeding for Rain. Though perhaps not an "industrial use," salt has been used to "seed" clouds to produce rain in desert areas.